Life sciences
Filters
Showing results for

Real-world data trends 2026: The shift to quality and AI precision
Key article takeaways

MarketScan 2025: Top achievements in real-world data and analytics
We started the year off talking about health economics and outcomes research (HEOR),...

Navigate New FDA Guidance on Real-World Evidence with MarketScan
The U.S. Food and Drug Administration (FDA) has formally acknowledged the pivotal...
How MarketScan data informs flu prevention strategies
Last week was National Influenza Vaccination Week (NIVW), and it is an important...

How real-world evidence from MarketScan supports smoking cessation strategies for COPD
Smoking remains the leading cause of chronic obstructive pulmonary disease (COPD),...

Unlocking insights: How MarketScan data transforms diabetes care and costs
November marks Diabetes Awareness Month—a time to spotlight the growing impact of...

Healthcare data took center stage at ISPOR 2025
It’s barely been one month since ISPOR 2025 and we are still amazed at the positive...

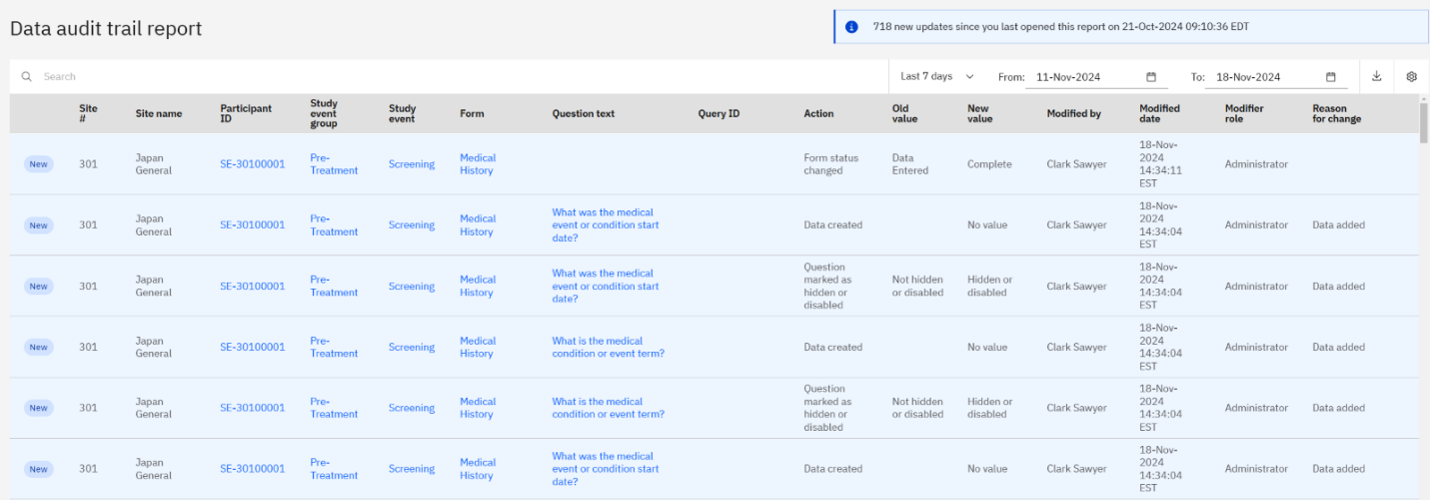
Zelta user success stories: Less stress, lower database costs, and fast-tracked clinical trials
Read about how Zelta brings database builds in-house, fast tracks clinical trials...
Zelta ranks as Major Contender in Everest Group’s 2024 Clinical Data and Analytics PEAK Matrix report
We’re excited to announce that Zelta has been positioned as a Major Contender in the...
Let the use case decide: The Zelta way on AI and supervised machine learning
For decades, when people thought of artificial intelligence (AI) what usually...

Five questions to ask before choosing a real-world data provider
When you’re working with real-world data (RWD) in a clinical trial setting, you must...

Zelta user success stories: Faster timelines, cleaner data, in-house flexibility
This month we’re profiling just a few of the CROs, sponsors, biotech, and pharma...

Mortality data: Driving health research forward
Learn how the MarketScan Mortality Database can help generate insights, examine...
The missing puzzle piece: Linking MarketScan claims data with Veradigm EHR data
This new healthcare database unlocks the whole picture of patient care at an...

Zelta ranked as a Leader in Everest Group’s 2024 EDC Products PEAK® Matrix Assessment
We’re excited to announce that Zelta has been officially ranked as a Leader in the...

Gene therapy: How Veristat cut trial database costs by 30%
This piece originally appeared in Pharmaceutical Technology. The gene therapy...

Optimize sample size and observation time: A case for employer-sourced administrative claims data
Closed administrative claims databases capture the complete range of healthcare...

Untapped opportunities: Fulfilling the promise of decentralized clinical trials
This piece originally appeared in Pharmaceutical Technology. The uptake of...

4 ways the vaccine rush is defining infectious disease trends for CROs in 2024
This piece originally appeared on Pharmaceutical Technology. The global healthcare...

Three real-world data research and regulatory trends to watch in 2024 and beyond
The Professional Society for Health Economics and Outcomes Research, known as...

The future of Zelta on display at the 2024 User Conference
That’s a wrap on the 2nd annual Zelta User Conference! We had a wonderful,...

Oncology in 2024: The clinical trial trends reshaping the role of CROs
This piece originally appeared on Pharmaceutical Technology. Oncology is the most...

Behind the posters: Exclusive insights from MarketScan researchers
Our MarketScan clients and researchers are fueled by curiosity and a passion to...

Measure the impacts of patient productivity loss and caregiver burden with real-world data
When patients are struck with illness, their quality of life and well-being are of...

Four ways a holistic ecosystem for clinical trials can help boost ROI
This piece originally appeared on Clinical Leader Clinical trials are complex and...

Advance rare diseases research with closed claims real-world data
MarketScan real-world data has been used to assess illness burden of rare diseases,...

Zelta surpasses 4,000 clinical trial studies
February 2024 marked a major milestone for Zelta: the platform has now hosted over...

What’s more important: Real-world data quality or speed?
Here is what executives from biopharma and medical device companies had to say...
Building the next generation of MarketScan: A year in review and what’s in store
About a year and a half ago, we said goodbye to IBM Watson Health and set off on an...

Can Your Clinical Trial Design Handle Mid-Study Changes?
As clinical research advances, the pharmaceutical and biotech industries...

Zelta expands its clinical trial ecosystem with a new partnership
At Zelta, we understand that collaboration is the name of the game when it comes to...

Lifesaving oncology research deserves cutting-edge solutions
The race to develop novel, advanced therapeutics for cancer patients is fast and...

New report confirms Zelta by Merative is a Major Contender
Merative has been named a Major Contender in Everest Group’s Life Sciences Clinical...

Looking beyond the bump with real-world evidence
Recent moves by the U.S. Federal Drug Administration (FDA) to open clinical studies...

Three years after COVID-19, the conversation around DCTs is shifting again
While interest in decentralized clinical trials (DCTs) has been heating up lately,...

The disconnect between clinical trial technology and legacy habits
In today’s age of cloud computing and data integration solutions, there are simple...

Merative Clinical Development has a new name
General Manager Jennifer Duff describes what the new name means for Merative’s...

Focused innovation will deliver steady progress in health and social care
Merative is taking a product-focused approach to solve real-world problems for our...

Introducing MarketScan WorkSpace: Move with speed, scale with ease, lead with confidence
In today's world, data is ubiquitous. From social media platforms to online...

Questions to ask when managing drug shortages
Drug shortages are nothing new, but the COVID-19 pandemic exacerbated this...

Connecting with the clinical development community
The time it takes to design, launch and complete clinical trials is a problem many...

Will DCTs finally democratize clinical trials?
Zelta Product Leader Walker Bradham and Senior Product Manager Wes Fishburne...

Cardiovascular image and data management: A technical and clinical evolution

COVID-19 pandemic and patient centricity in clinical trials
Jennifer’s career as a clinical data management and clinical development executive...

Is real-world data the real deal?
Real-world data is Liisa’s world. After receiving her PhD in health policy and...

Beyond KLAS rankings: building client relationships at Merative

Decentralized clinical trials are closer than you think
Jennifer’s career as a clinical data management and clinical development executive...