ZELTA RTSM
Streamline clinical trial execution with Zelta RTSM

News
Over 4,500 clinical trials have been hosted on Zelta. Hear from our users why Zelta has become the platform of choice for their clinical research. Learn more.
OVERVIEW
Streamline your trials...
...with only the modules you need.
Clinical operations teams do critical work to ensure that trials run smoothly, stay on schedule and on budget, and generate reliable data that yields results and meets regulatory compliance.
Zelta streamlines these processes to give you greater control and confidence over your clinical trials. Choose only the modules you need for each study — regardless of complexity or stage — from a fully integrated, cloud-based electronic data capture platform.
...with only the functionality you need.
With Zelta’s advanced randomization and trial supply management (RTSM) functionality, you'll gain even greater control over your clinical trials. Our predictive resupply algorithm ensures inventory never runs out or exceeds demand, and automates the dispensing and dosing of investigational products. This reduces waste, enhances compliance, and keeps every aspect of your trial aligned.
-
RTSM
-
Globalization
-
Endpoint adjudication
-
eLearning
-
CTMS (powered by BSI)
-
eTMF (powered by BSI)
Benefits of a single, integrated EDC and RTSM solution
Clinical trial supply management
Forecast trial site inventory with predictive resupply algorithms, support variable dosing schedules and inventory management, and empower stakeholders making real-time supply chain decisions.
Real-time data flow
Intelligent and integrated CTMS, eTMF, and CDMS platform helps enable real-time data flows and keep study timelines on track.
Simplify enrollment and randomization
Streamline trial operations and reduce timelines and costs by eliminating need for complex cross-platform, third-party integrations.
Video
How Zelta EDC has changed the game on bringing studies to go-live


Manage clinical trials with a flexible and comprehensive RTSM solution
-
Simplify enrollment and randomization with a single, integrated EDC and RTSM platform.
-
Leverage KPI-based real-time reports to assist with managing your clinical research.
-
Flexible and adaptive supply management functionality supports needs of decentralized clinical trials.
-
Incorporate real-time data directly into Zelta's EDC.
.png?width=552&name=IMG-Zelta-Web-RTSM-01-1104x552%20(1).png)
Optimize clinical trial supply strategies with predictive algorithms
-
Achieve accurate drug dispensing and investigational product accountability with Zelta’s real-time trial supply management powered by predictive resupply algorithms.
-
Supports other resupply capabilities such as floor ceiling.
-
Oversee depot coordination and clinical site resupply with precision-focused workflows.
-
Enable automated inventory management to streamline processes, save time, reduce costs, and make sure investigational products go to the right place at the right time.
-
Support static and dynamic randomization schemas, including stratified, block, crossover, and replacement randomization.
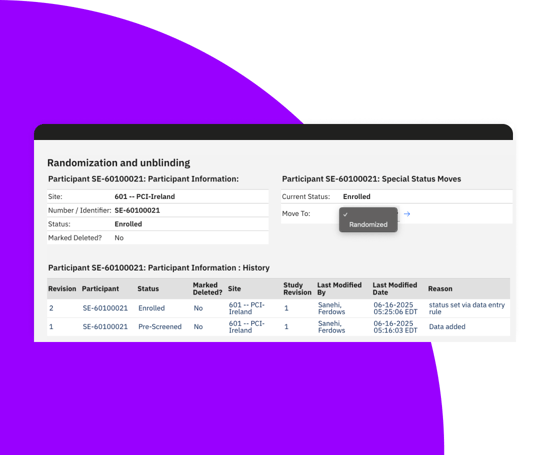
Efficient RTSM system for complex study designs
-
Manage randomization of participants with automatic and manual assignment options.
-
Support diverse protocol needs, including multiple treatment arms, adaptive trial design, treatment assignment, and drug supply and inventory management.
-
Adapt to variable dosing schedules and multiple treatment arms.
-
Track batch identifier, sequence number, and expiry as blinded information ensuring data integrity and compliance.
-
GCP (Good Clinical Practice) 21 CFR Part 11 compliance ensures secure clinical data management.
-
Export sensitive unblinding data by allowing users with appropriate permissions to choose to include unblinded information in exports.
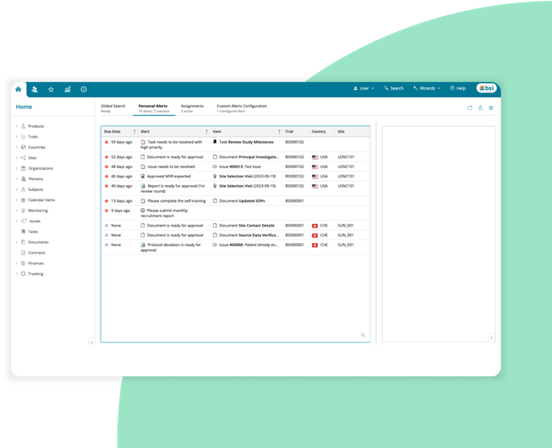
Add-on clinical operation capabilities as you need them
-
eLearning — Manage training to ensure regulatory compliance.
-
Globalization — Zelta supports over 70 languages and dialects, in more than 100 countries, so users can interact in their local language.
-
CTMS — Oversee end-to-end local, regional, and global clinical trials.
-
eTMF — Flexible electronic Trial Master File (eTMF) for managing regulatory study documents.
What you get with Zelta
Discover Zelta’s other fully built-in EDC modules
Clinical data management and acquisition
- Electronic data capture
- Medical coding
- Local labs
“It’s a huge benefit and time saver. The methodical flow of the screen makes for very effective data entry, “But we also measure success by the overall preventable error rate of a database. It’s important for the end-user to operate a user-friendly and intuitive EDC system like Zelta so data entry is efficient. Having the ability to correct those data entry errors in real-time saves a lot of time and money.”
Krista Dahling, Supervisor of Clinical Data Management, Solventum
“This partnership with Zelta by Merative is all about life science organizations maximizing their clinical trial management processes. BSI understands there are many moving parts when it comes to a clinical trial execution and we believe a complete, integrated, and intelligent CTMS, eTMF and CDMS platform is what will help organizations allow for real-time data flow and keep their studies on track.”
Jan Nielsen, Community Manager, BSI Life Sciences
“Despite the trial’s level of complexity, our sites have been able to conduct it successfully, thanks to the Zelta solution.”
Program Clinical Data Manager, Worldwide Clinical Trials
RESOURCES
Dig deeper into Zelta EDC
Introduction to Zelta
Explore how the Zelta clinical trial solution offers comprehensive, intuitive features to help you build and manage clinical trials across all phases and levels of complexity.
How a holistic ecosystem for clinical trials can help boost ROI
Zelta enables pharmaceutical and biotech companies to shift from custom, siloed solutions to broader, more holistic ecosystem.
Zelta ranked as EDC Leader
See why Zelta was ranked as an industry Leader in the Everest Group’s 2024 EDC Products PEAK® Matrix Assessment and how our EDC platform is ideal for CROS and sponsors.
Ready to talk?
Speak with a Zelta clinical development expert or see a solution demo.
