ZELTA ECOA
Seamlessly collect clinical research data directly with Zelta eCOA

News
Over 4,500 clinical trials have been hosted on Zelta. Hear from our users why Zelta has become the platform of choice for their clinical research. Learn more.
OVERVIEW
Unified EDC with built-in eCOA for seamless workflows
Zelta is an all-in-one platform with eCOA fully built into our electronic data capture (EDC) system. With single sign-on access, Zelta enables cohesive workflows, efficient data management, and improved data quality for clinical trial teams.
We simplify clinical trials by supporting both participants and trial managers. Our advanced electronic patient-reported outcomes (ePRO) and electronic observer reported outcomes (eObsRO) capabilities ensure accurate, reliable data collection through diaries, questionnaires, surveys, and assessments — anytime, anywhere. This reduces barriers for users, enhances patient engagement, and streamlines data collection.
Zelta promotes patient-centric clinical trials with intuitive tools that minimize administrative workload, improve patient adherence, and enhance the user experience. By prioritizing ease of use, Zelta helps create clinical trials that truly work for patients and researchers alike.
-
eCOA/ePRO
-
eConsent
Patient experience
Convenient, direct data entry into Zelta EDC with intuitive navigation for participants via computer, tablet, or smartphone.
Customize data management and design
Flexible eCOA data collection design options support user experience and data integrity.
Clinical operations
Participants are onboarded with a unified clinical data management platform, reducing the burden of site technology and compliance monitoring.
Video
The benefits of a built-in ePRO module
Mark Corder, clinical data manager at Datafy, talks about the benefits of Zelta's built-in ePRO module and how leveraging the platform's all-in-one capabilities mean they're not spending time finding disparate pieces of data from different sources and vendors, and how this approach reaps enormous ROI on cost and time savings.

Deliver the best experiences for clinical trial participants and site personnel
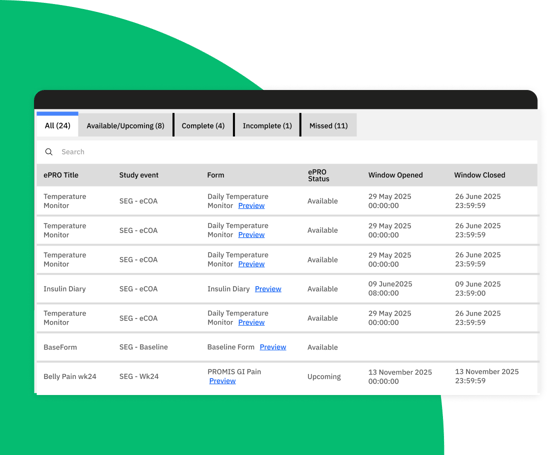
Built-in ePRO for seamless and efficient clinical trials
-
Zelta’s eCOA solution is built directly into the platform. Enjoy single sign-on access for cohesive workflows, efficient data management, and improved data quality for clinical trial teams.
-
Design and manage ePRO data collection forms, schedules, and rules directly within Zelta EDC, ensuring seamless setup for your team.
-
Access Zelta ePRO anytime, anywhere with compatibility across browsers, iOS, and Android devices for ultimate flexibility.
-
Customize workflows to the specific needs of your trial.
-
Convenient, real-time patient-reported outcomes that deliver actionable insights and improve participant adherence.
-
Zelta eCOA/ePRO captures data directly from clinical trial participants.
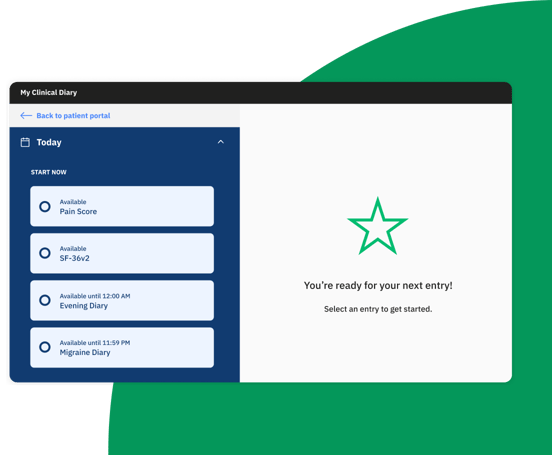
Enhance participant experience with real-time patient-reported outcomes
-
Personalize engagement with participants and caregivers through real-time notifications and multilingual support.
-
Optimize regulatory compliance while ensuring secure handling of patient data.
-
Choose between bring your own device (BYOD) or issued-device compatibility for hybrid trials, depending on your needs.
-
eCOA forms can be saved as drafts for the patient to complete when convenient for them.
-
Replace traditional paper-based methods with an intuitive ePRO solution available on mobile devices to make questionnaires and data entry simple and stress-free for participants.
.png?width=552&name=IMG-Zelta-Web-ePRO-03-1104x552%20(1).png)
Optimize data management and site experience with ePRO
-
Optimize data management in clinical trials with ePRO solutions that enhance data quality, patient compliance, and safety.
-
Improve data quality and streamline electronic clinical outcome assessment eCOA processes.
-
Empower decision-making with real-time data on patient-reported outcomes and compliance feedback.
-
Simplify data collection with a BYOD approach, issued devices, and flexibility for hybrid trials.
-
Ensure seamless data entries and regulatory compliance with accurate timestamping and comprehensive audit trails.
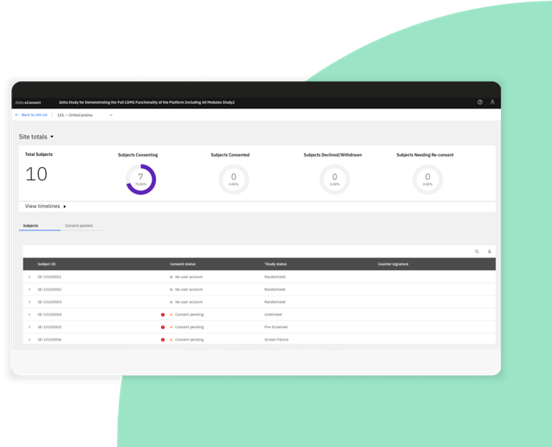
Zelta eConsent: Quickly consent participants wherever they are
-
Maximize site efficiency and remote management of participant consent for clinical trials.
-
Manage eConsent directly from Zelta’s electronic data capture system, alongside the eCOA/ePRO module.
-
Track amendments, consent status, and timelines in a dedicated dashboard.
-
Increase participant convenience with single sign-on access to both eConsent and eCOA/ePRO.
eCOA and ePRO unleash the benefits of decentralized trials
Decentralized clinical trials (DCTs) offer several benefits for CROs and sponsor companies, including reduced need for site travel, lowered trial costs, and better patient-reported outcome data for analysis.
Enabling trial participants to report outcomes via ePRO or using eConsent can greatly improve the patient experience, boost recruitment for trial managers, and enable real-time data capture. The Zelta platform – including eCOA, ePRO, eConsent, and other modules – has been used to successfully build, run, and support over 250 hybrid trials or DCTs.
What you get with Zelta
Discover Zelta’s other fully built-in EDC modules
Clinical data management and acquisition
- Electronic data capture
- Medical coding
- Local labs
Clinical operations
- RTSM
- Globalization
- eLearning
- Endpoint adjudication
- CTMS (powered by BSI)
- eTMF (powered by BSI)
“EDC-embedded ePRO is an incredibly valuable tool we’ve added to Alimentiv’s toolbox. It allows us to have data readily available. In our therapeutic area, we’re highly reliant on patient-reported data to support endpoints. With Zelta EDC, there’s no sync, no lag, the data is already committed directly to the EDC, and that helps us be one step quicker to a decision.”
Chris Walker, Senior Director of Clinical Data Sciences, Alimentiv
“We’re using Zelta ePro for various studies. What I like about it the most is having all of that in one place. We don't have to go to disparate data sources, vendors, compare that data back into EDC to make sure that our dates are matching. We’re just basically looking to see who is the next subject, when is their ePRO due, and reminding clinical, make sure they get it done.”
Mark Corder, Clinical Data Manager at Datafy
RESOURCES
Explore what Zelta EDC has to offer
Fast tracking clinical trials with ePRO and self-service
Find out how Zelta’s ePRO capabilities and self-service support accelerate clinical trial timelines with dozens of patient-reported outcomes.
Decentralized clinical trials are closer than you think
Read more on how DCTs give clinical trial developers the opportunity to create more patient-centric designs – better and faster than before.
How SaaS platforms are breaking the mold for clinical trials
Read about how Zelta’s built-in RTSM, eConsent, and ePRO/eCOA systems can reduce inefficiencies for clinical trials of any therapeutic areas phase, or complexity.
