ZELTA EDC: ELECTRONIC DATA CAPTURE
Electronic data capture to improve study builds
Zelta’s user-friendly EDC system helps build and execute your clinical trials with improved efficiency, flexibility, and speed.

News
Over 4,500 clinical trials have been hosted on Zelta. Hear from our users why Zelta has become the platform of choice for their clinical research. Learn more.
OVERVIEW
Zelta Electronic Data Capture
Zelta’s electronic data capture system is a flexible and secure eClinical solution for executing clinical trials, including decentralized trials solutions. Used in more than 4,500 clinical studies from startup to submission, across all phases, including over 500 phase III trials and 23 therapeutic areas, Zelta makes it easier to execute completed clinical trials in EDC.
-
Electronic data capture
-
Medical coding with AI
-
Local labs
Flexibility and scalability
Execute on a more flexible cloud-based clinical data acquisition and data management system, supporting study sites and participants that span more than 100 countries and over 75 languages and dialects.
First-time right data entry
Reduce programming and optimize workflows for faster first-time right clinical trial data collection using real-time data validation.
Supervised machine learning
Use supervised machine learning and context-based logic to build studies faster.
Let the use case decide
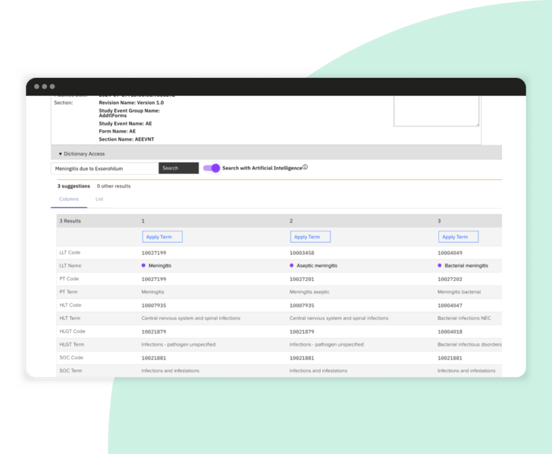
AI and supervised machine learning for EDC in clinical research
-
Take advantage of point-and-click electronic data capture designer and automatic design validation.
-
Streamlined coding process enhances accuracy and supports regulatory compliance.
-
Manual coding searches reduced by >50% with Medical Coding with AI directly integrated into Zelta’s clinical data management and EDC platform.
-
Use supervised machine learning to streamline the manual process of annotating CRFs with CDASH, allowing for quicker clinical study startup.
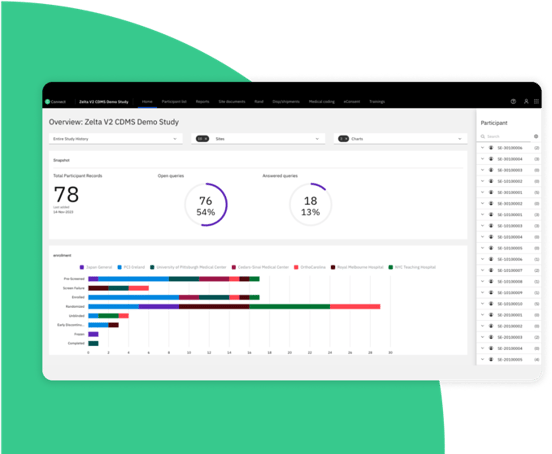
Simplify the trial experience: for your teams, sites, and participants
-
In-app, KPI-based dashboards and visualizations generate real-time insights to monitor trends, identify bottlenecks, and track other clinical study and dataset metrics.
-
Immediate feedback during data entry and clinical data acquisition, including real-time edit checks and auto calculations, reduces queries and delivers first-time right data.
-
ODM regulatory compliance means faster data integrations and compliant data outputs for any clinical study.
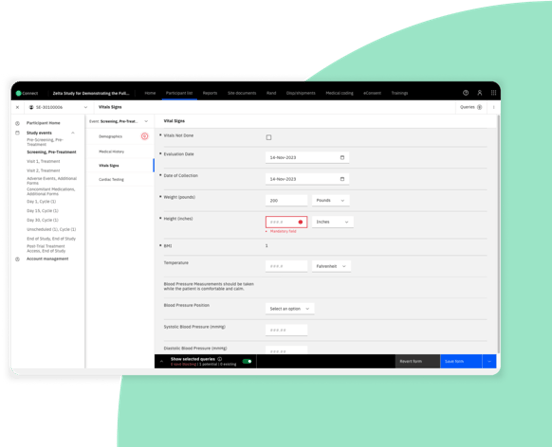
Built-in optimized workflow solutions
-
Eliminate building every cycle/visit combination and take advantage of repeating event groups to streamline your electronic data capture workflow.
-
Never forget the basics with EDC system level null and future date checks in place from the start.
-
Reduce the time required to design and standardize CRFs, allowing for quicker study startup.
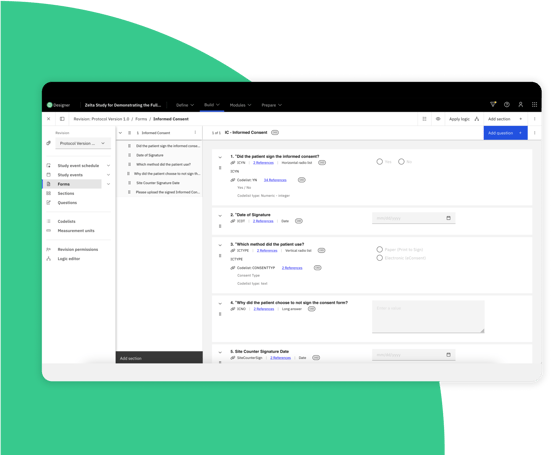
Fewer disruptions from mid-study changes
-
Quickly and easily make changes and deploy mid-study updates automatically.
-
Reduce site and participant downtime from days or weeks to minutes.
-
Built-in error checking helps promote confidence in releasing clinical study amendments.
-
<30 minutes for data entry lock to deploy mid-study updates in Zelta’s Electronic Data Capture system.
Customizable clinical data acquisition and management functionality
-
Manage local labs at a program level, reducing redundant work and ensuring data quality and accuracy across trials.
-
Integrate with third-party solutions, CTMS, RTSM, or CDMS with a built-in point-and-click tool.
-
View up-to-the-minute study data and easily navigate to points of interest within the data.
-
Interact with discrepancies in real-time and action them directly from the eCRF.
-
Achieve profound, comprehensive insights from flexible, responsive, and embedded reporting.
Video
Flexibility to build EDC clinical studies in-house
Hear from Ray Carlburg, Associate Director of Data Management at Poseida Therapeutics, on how the flexibility of the Zelta electronic data capture platform empowered his team to build clinical studies in-house -- giving them greater control over resourcing and clinical trial timelines, eliminating communication challenges, and achieving significant time and cost savings.

Ready to talk?
RESOURCES
Dig deeper into Zelta’s data management modules
Zelta ranked as EDC Leader
See why Zelta was ranked as an industry Leader in the Everest Group's 2024 EDC Products PEAK® Matrix Assessment.
Launching over 250 databases on a flexible, customizable EDC platform
Watch to see how Alimentiv is leveraging a flexible EDC platform with a built-in ePRO.
Adaptive EDC Clinical Trials
Explore how sponsors, CROs, and technology partners can leverage Zelta to simplify adaptive trials.
The 24-hour study build
Zelta envisions a future where building, testing, and launching a clinical study can happen in just 24 hours. The technology now exists to automate and streamline these processes. ...
Make medical coding easier with Zelta Electronic Data Capture
Correctly coding patient data is crucial to the success of a clinical trial, but the process can be tedious and error-prone. Learn how Zelta’s clinical trial platform uses AI to ...
Gene therapy: How Veristat cut trial database costs by 30%
Gene therapy clinical trials are held back by high costs and study complexity. Learn how Veristat reduced their trial database costs by 30% with data management support from Zelta.
Ready to talk about your clinical data management needs?
Speak with a Zelta clinical development expert or see a solution demo of Zelta Electronic Data Capture.
