Zelta now the host platform for over 4,500 clinical studies
Zelta users share their stories of simple user experiences, time and cost savings, and the convenience of built-in ePRO and RTSM for their clinical studies.
2025 marks two major milestones for Zelta. Earlier this year, we celebrated our 15th anniversary – 15 years of providing an all-in-one electronic data capture, RTSM, eCOA/ePRO clinical data management platform that has helped to drive bold research in thousands of clinical studies around the world. This month, we celebrate our second milestone: as of today, the Zelta platform has officially hosted over 4,500 clinical trials!
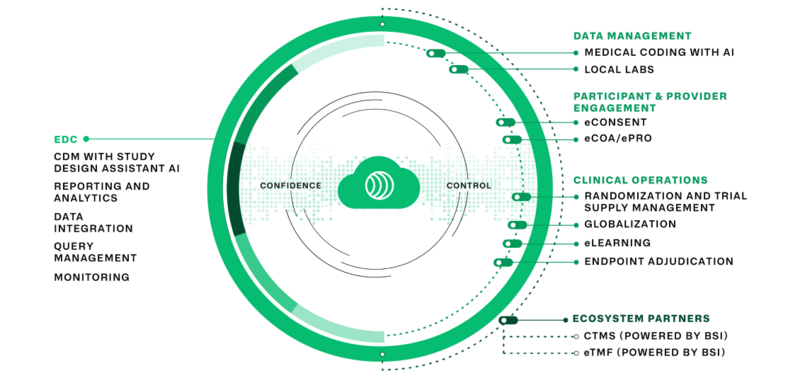
Since 2010, Zelta has strived to create a clinical data management platform that is easy to use, built on one code base which means it is a truly integrated solution, and can scale to its users’ clinical trial needs – whether it’s supporting studies across all phases (including Phase III and IV trials, pivotal trials, and registries), or providing electronic data capture, eCOA, ePRO, eConsent, RTSM, and medical coding with AI all in one platform – whenever you need it, however you need it.
Thanks to years of innovations from our team and collaboration with over 200 CRO, biopharma, medical device, and academic research organizations, the Zelta platform has become exactly that.
4 hallmarks of success across Zelta’s 4,500 clinical trials
The 4,500 studies hosted on Zelta are truly global – spanning over 100 countries and more than 75 languages and dialects, with trials of all phases and levels of complexity. Hosting your clinical trial on Zelta means enjoying a wide-range of benefits, from simple user experiences to significant cost and time savings to the convenience of having built-in modules like ePRO and RTSM = zero integrations!
Don’t take my word for it; we like to let our users speak for themselves about the value Zelta brings.
Saving time
“We were able to build our first study in Zelta in just two weeks... The time savings have been great for us and it has been really easy to navigate the system.”
-- Jennifer Tucker, Former Director of Data Management, Alira Health
“It’s a huge benefit and time saver. The methodical flow of the screen makes for very effective data entry. But we also measure success by the overall preventable error rate of a database. It’s important for the end-user to operate a user-friendly and intuitive EDC system like Zelta so data entry is efficient. Having the ability to correct those data entry errors in real-time saves a lot of time and money.”
--Krista Dahling, Supervisor of Clinical Data Management, Solventum
“We’re spending less time in repetitive review and spending more time bringing actual value in by being able to monitor what’s going on.”
--Mark Corder, Clinical Data Manager at Datafy
Easy user experience
“Trust me when I say, our team was very relieved when we brought data management in-house to have more control over data collection. Using Zelta has been great, it has eliminated the stress factor we had with our last vendor. Now we’re able to build and manage databases to our satisfaction and Zelta makes that possible.”
-Tserundede Ekwue, MD, Sr Manager, Clinical Data Management, Clinical Affairs, Sysmex
“Building cleaner databases within Zelta has been an intuitive process. The contrast in data cleanliness between a Zelta-designed database and the databases we inherited from other vendors has been night and day.”
-- Dimitri Fitsialos, CEO, Integrated Therapeutic Solutions
“The first thing I think of when I think of Zelta is it’s user friendly. Easy to use, easy to build.”
--Katrina Riggs, CEO, Fast-Track Drugs & Biologics, LLC
The benefits of built-in ePRO and RTSM
“EDC-embedded ePRO is an incredibly valuable tool we’ve added to Alimentiv’s toolbox. It allows us to have data readily available. With Zelta, there’s no sync, no lag, the data is already committed directly to the EDC, and that helps us be one step quicker to a decision.”
--Chris Walker, Senior Director of Clinical Data Sciences, Alimentiv
“One of [Zelta’s] key strengths is its all-in-one design, seamlessly integrating multiple solutions within a single EDC platform.”
--Aleksandra Bąk, Data Manager, KCRI
“The Zelta RTSM system in particular has been game-changing in the way that we can configure and be able to adapt for studies. If you have a protocol update or something that adds, say, an extra level of stratification or anything like that, being able to configure that has been really easy, which is not something you always see.”
-Catie Upton, Data Analyst for InFocus Digital
Having a 24/7 Zelta support team available as you need them
“I exclusively use Zelta for the last 12+ years for building studies and managing clinical studies. I reach out to the Zelta team for any hiccups that I face. They respond within a couple of hours and the solution they give is spot on. I really appreciate their support.”
--Natesan Elango, Fellow, Clinical Affair/Medical Affairs, Endologix
“Zelta’s support group is fast and they are correct. They get you the answer that you need, and they do it in a fantastically quick turnaround.”
--Mark Corder, Clinical Data Manager at Datafy
Why choose Zelta for your next clinical trial
- All-in-one EDC platform capabilities, with integrated CTMS and built-in RTSM, ePRO, and other modules = ZERO INTEGRATIONS
- Highly configurable, easy-to-use, low-code platform that lowers the barrier to entry for users = DON’T NEED DATABASE PROGRAMMERS
- Extensive network of partners and collaborators that provide Zelta users with multiple options to choose from = OPERATE WITH AN ECOSYSTEM OF BEST-IN-CLASS PARTNERS
- A flexible suite of offerings that can be customized to the specific demands for trials of any phase or level of complexity, backed by a robust 24/7 customer support team that will address and help remediate any challenges you may face as quickly as you need = BEST-IN-CLASS SUPPORT
Build your next clinical trial with Zelta
Over 4,500 (and counting) clinical studies can’t be wrong! There’s never been a better time to start your Zelta journey, especially as we continue to push into innovative new boundaries – such as our vision for making the 24-hour study build a reality.
See what hundreds of organizations like yours have discovered from building and running their clinical studies on Zelta: a time-saving, easy-to-use, enjoyable experience.
Unlock your own interactive Zelta demo to see for yourself how Zelta can help.
Ready for a consultation?
Our team is ready to answer your questions. Let's make smarter health ecosystems, together.
